The thymus is highly sensitive to acute injury such as the cytoreductive conditioning given pre-hematopoietic stem cell transplant (HCT). The thymus is capable of regeneration, however its reparative capacity and T cell productivity decline with age. This leaves HCT recipients vulnerable to relapse of malignancy and opportunistic infection - the leading causes of post-HCT mortality - during a prolonged period of lymphopenia. Better understanding the endogenous mechanisms by which thymus regeneration is regulated may inform therapeutic interventions to improve T cell reconstitution in these patients. Here, we report that HCT conditioning leads to rises in stimulatory cytokine IL-18, subsequent activation of NK cells and increased cytotoxicity, which aberrantly suppresses organ recovery.
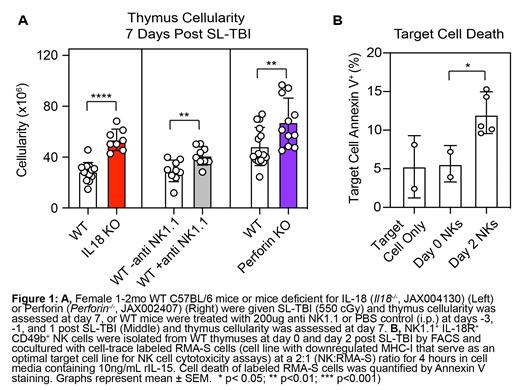
Our group has reported that HCT-conditioning by ionizing radiation leads to increased Caspase-1 mediated immunogenic cell death within the thymus (Kinsella 2023 BioRxiv). Consistent with the cleavage of Caspase-1, we found an increase in release of the inflammatory cytokines IL-1b and IL-18. Although mice deficient for IL-1b signaling receptor ( Il1r -/-) did not show any modulation in their ability to regenerate after TBI, we found that mice deficient for IL-18 signaling (Il18 -/-) exhibited increased thymic cellularity one week following acute damage by sublethal irradiation (SL-TBI) (Fig. 1A left). This led us to conclude that post-damage activation and release of IL-18 suppresses thymus regeneration.
We found that IL-18R was not expressed on most developing thymocytes and although a minority of thymic epithelial cells expressed the receptor, mice with a deficiency in IL-18R restricted to TECs ( Il18r1fl/fl:Foxn1-Cre +) showed no difference in regenerative capacity following conditioning. To rule out an effect on hematopoietic progenitors, for which IL-18R expression has been reported ( Silberstein 2016 Cell Stem Cell 6;19), we performed a competitive transplantation of Il18r1 -/- and WT bone marrow and measured T cell production, which showed no competitive advantage of Il18r1 -/- donor cells; this demonstrated that IL-18 does not directly regulate progenitor cells themselves, but rather, more likely acts via a bystander thymus-resident population. Within the thymus, IL-18R was expressed by highly radioresistant NK1.1 + NKT and NK cells. Depletion of both populations with anti-NK1.1 monoclonal antibody improved thymus cellularity in WT mice (Fig. 1A middle). Notably, NKT deficient CD1d -/- mice showed no defective repair which suggested that the depletion upon NK1.1 + cell depletion is largely mediated by NK cells. Depletion of NK1.1 + cells in Il18 -/- mice did not improve regeneration further, suggesting that NK-mediated suppression is IL-18 dependent. Given the known role of IL-18 in NK cell activation, we hypothesized that the HCT-conditioning resultant rise in IL-18 stimulates NK cells leading to “accidental” targeting of regeneration promoting cells. NK cell production of cytotoxic factors including IFNg, GzmB and Perforin increases following radiation conditioning, although only mice deficient for Perforin and not IFNg -exhibited improved thymus cellularity 7 days post HCT-conditioning (Fig 1A right). Further supporting this hypothesis, IL-18R +CD49b + NK cells isolated from the thymus 2-days post-irradiation induced more target cell death in head-to-head cytotoxicity assays compared to NK cells isolated pre-radiation (Fig 1B). Finally, we observe TEC downregulation of NK cytotoxicity inhibitory ligand MHC-I following radiation conditioning, suggesting that critical regeneration promoting stroma may be the targets of these activated NK cells.
These findings suggest that, while necessary for successful transplantation, conditioning regimens may induce cytotoxicity of bystander NK cells which suppresses thymus recovery. Furthermore, we implicate conditioning induced immunogenic cell death and increased IL-18 in the stimulation of these suppressive cytotoxic cells and suggest that therapeutically targeting IL-18 and NK cell cytotoxicity may improve T cell reconstitution post-HCT.
Disclosures
Iovino:Mustang Bio: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal